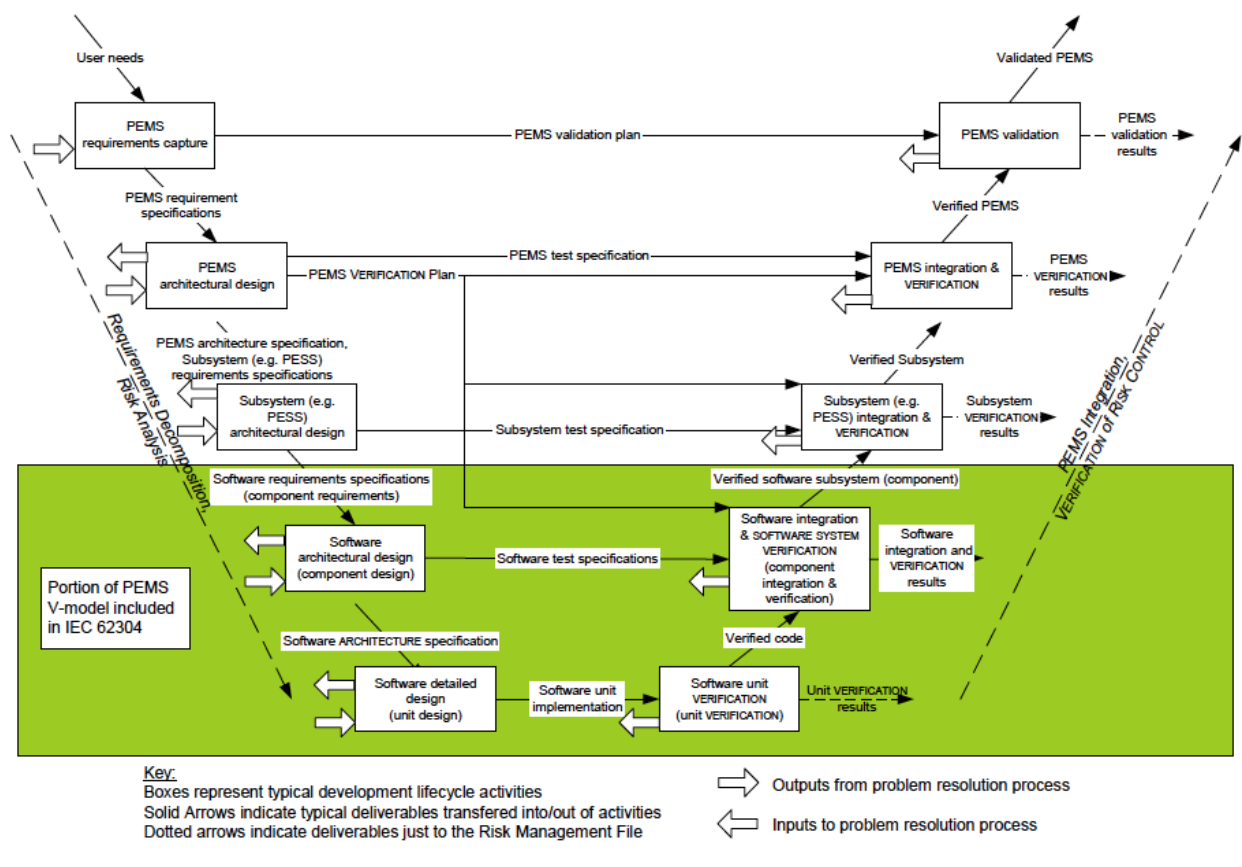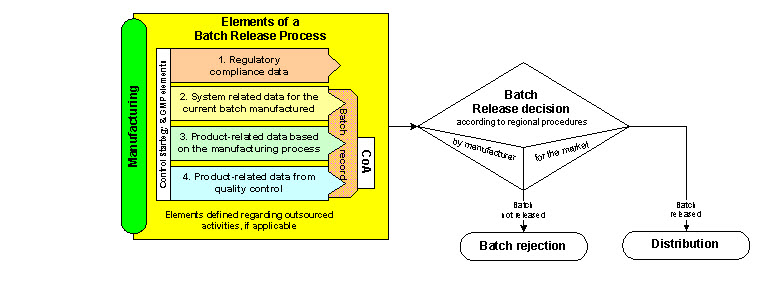
Q8, Q9, & Q10 Questions and Answers -- Appendix: Q&As from Training Sessions (Q8, Q9, & Q10 Points to Consider) | FDA
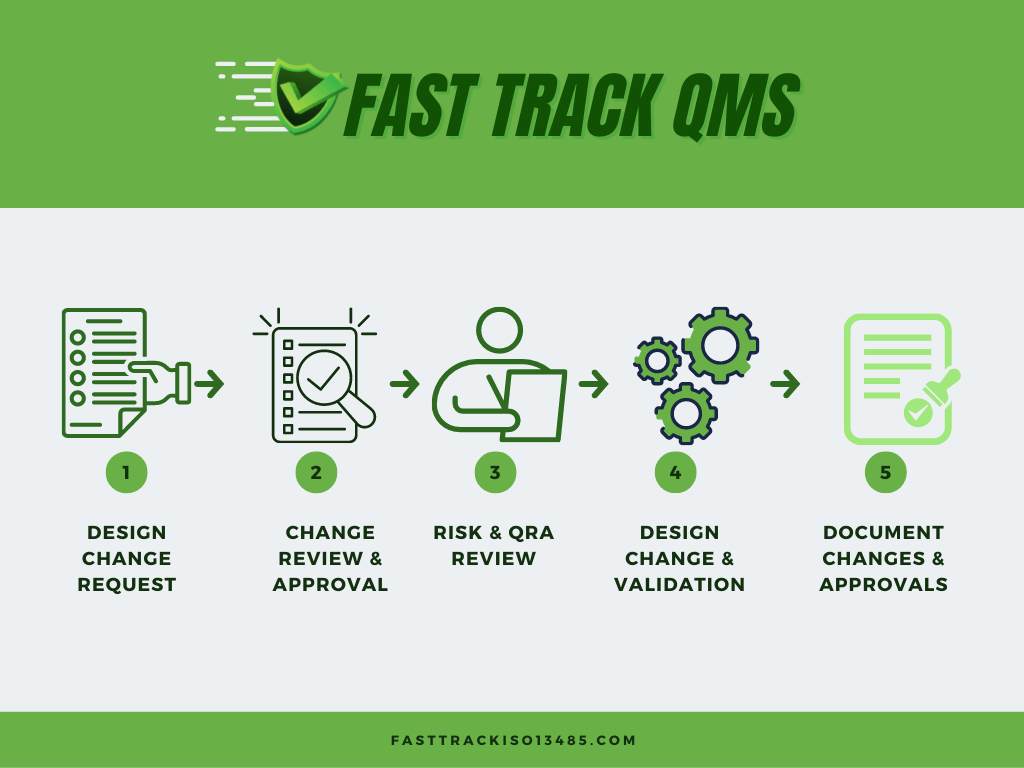
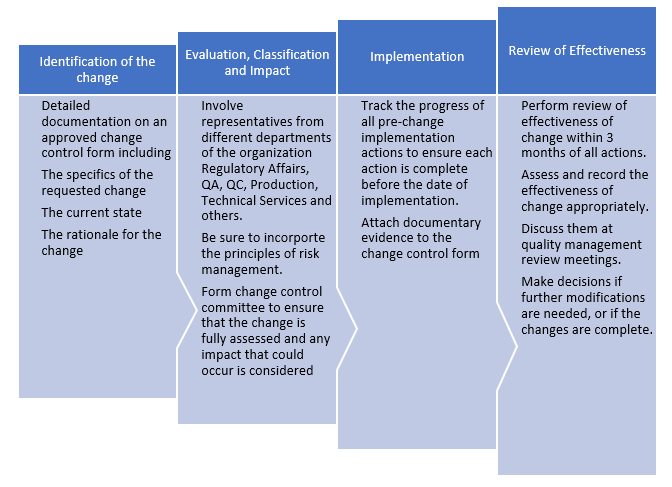
Fast Track ISO 13485 | How to Control Design Changes for your Medical Device and meet ISO 13485 requirements

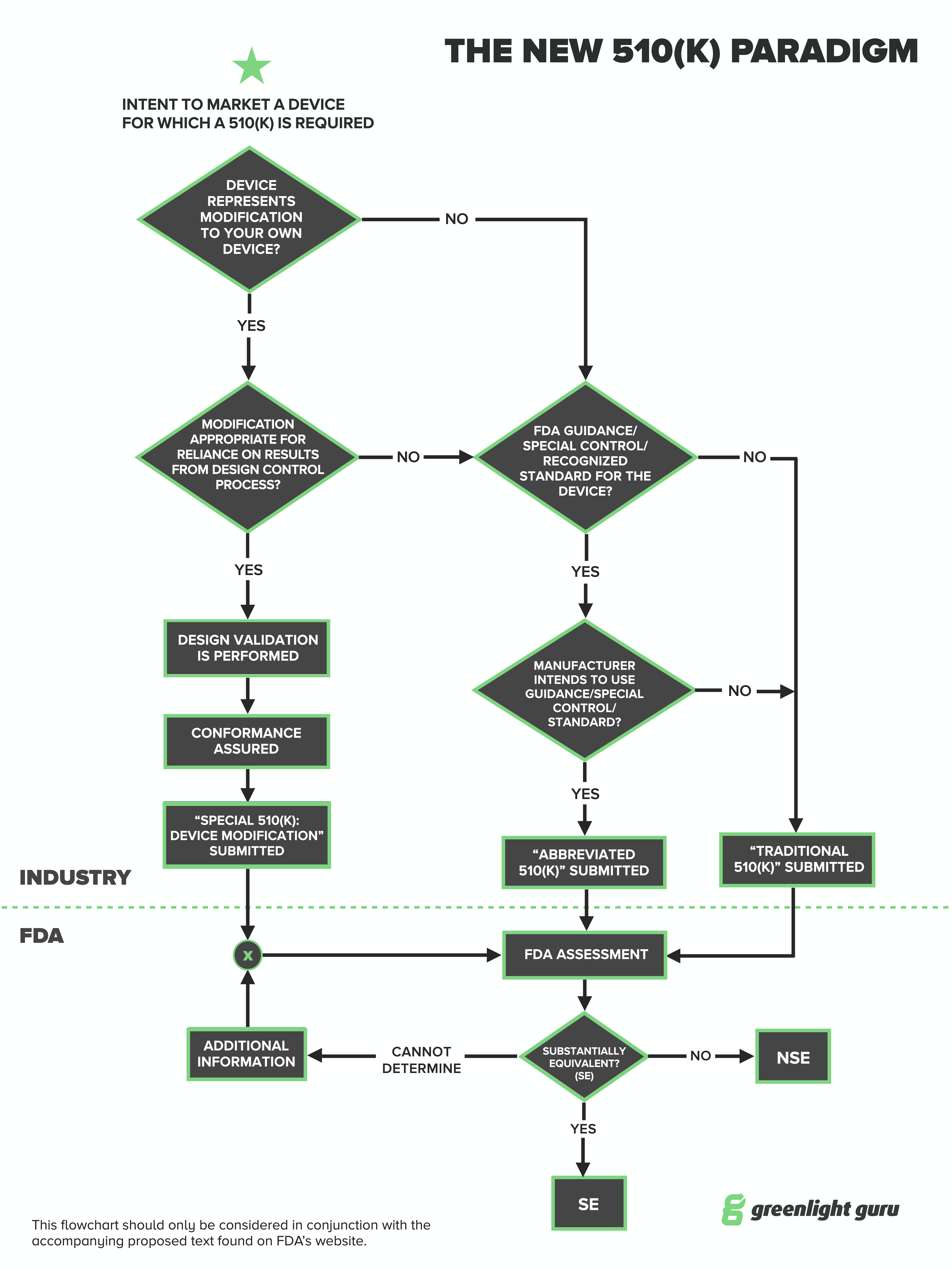
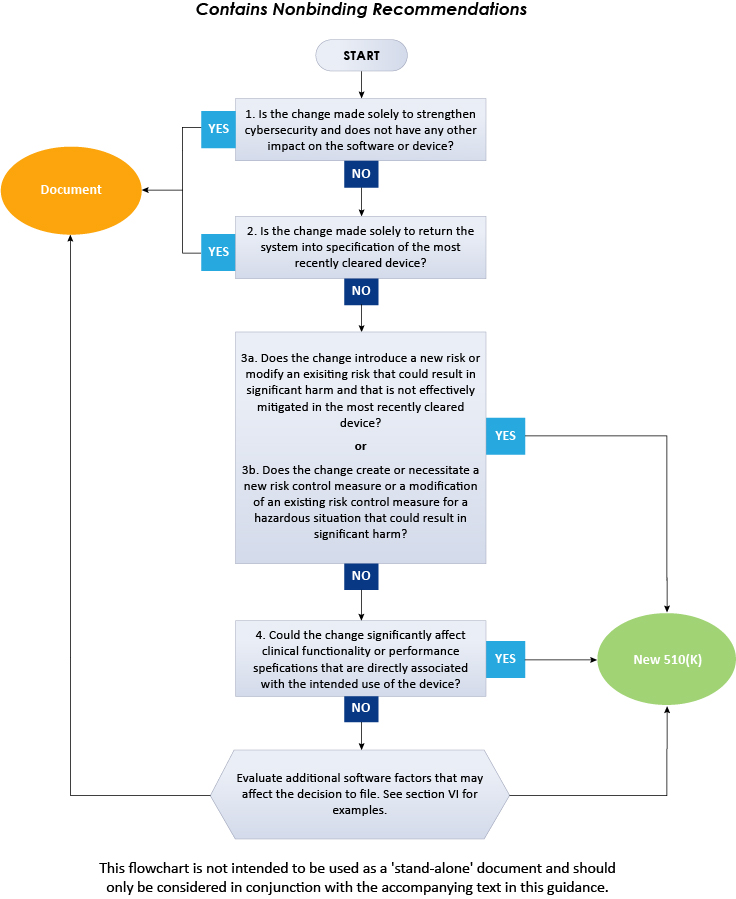
AI developers should build robust change control protocols despite absence of FDA guidance | BioWorld

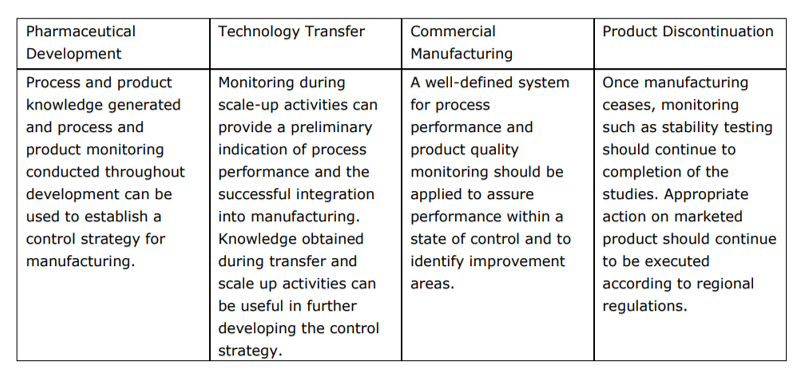
ICH Q12 Examples – Pharmaceutical Product Lifecycle Management Examples – FDA Guidance – Quality by Design for Biotech, Pharmaceutical and Medical Devices

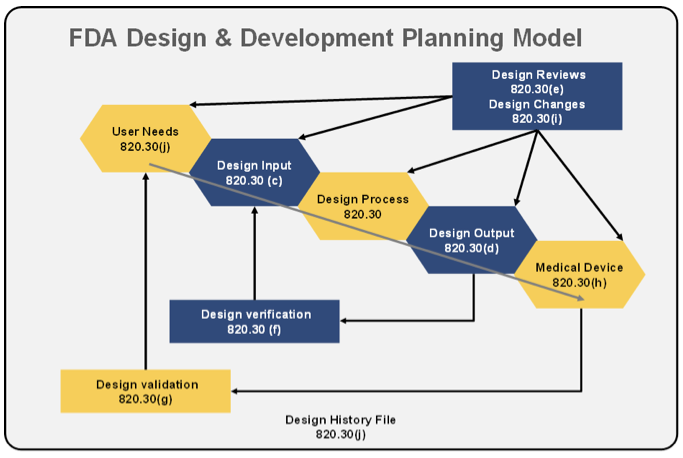





.webp)